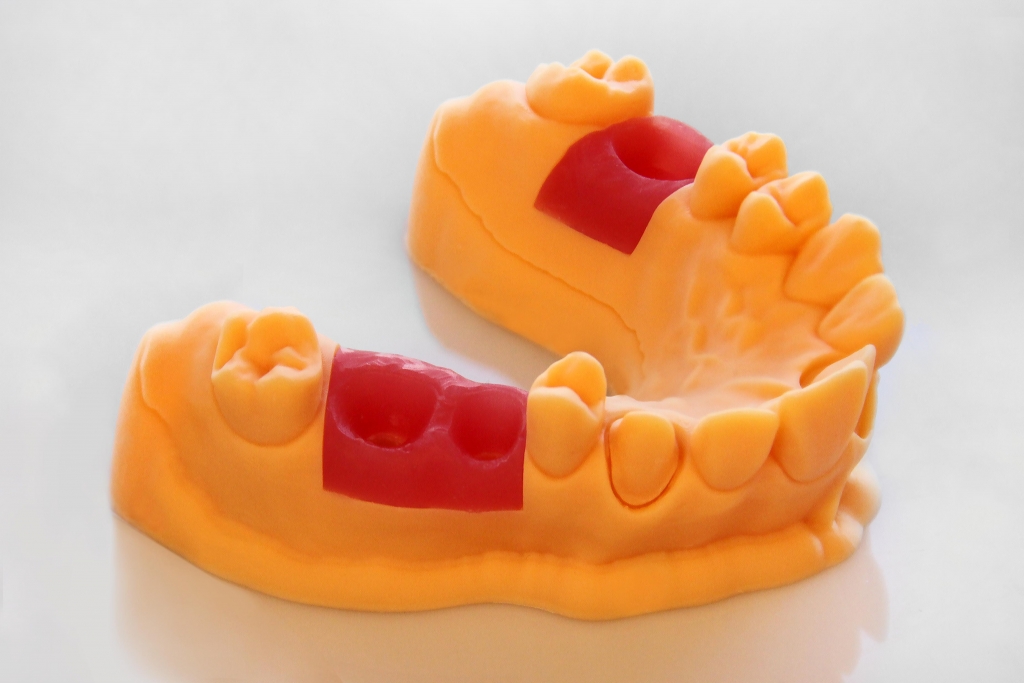
EnvisionTEC will announce the new E-Guard material for their 3D printers.
E-Guard is FDA-approved and was developed for dental applications. EnvisionTEC CEO Al Siblani about the new material:
We’re thrilled to be able to announce the latest addition to our innovative line of premium 3D print materials at the largest dental laboratory event in America. Our new material can be printed on our entire Perfactory® and Vida 3D Printer lines and is the perfect material for high throughput dental applications, providing on-site turnkey solutions for dental labs and offices. Although not yet available, we will also be previewing E-Gum material, our 3D printing solution to simulate the flexibility of the patient’s gum in flexible gingiva masks for integration into 3D printed dental models to adjust dental prostheses properly,” said Siblani.
Subscribe to our Newsletter
3DPResso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.