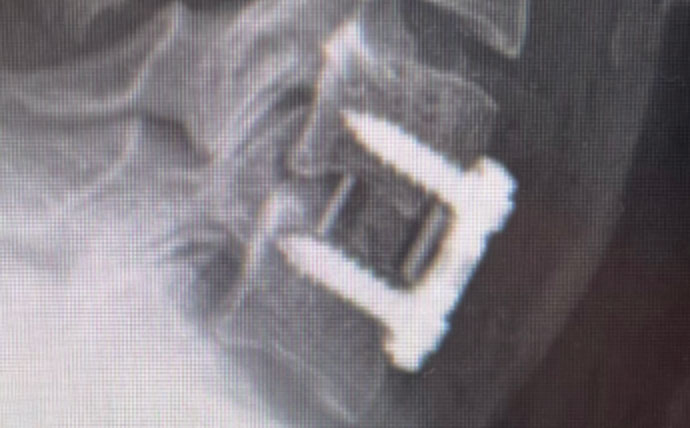
Technology company Curiteva announced a limited commercial release for its recently FDA-approved Inspire Porous PEEK HAFUSE Cervical Interbody System.
Curiteva pioneered and received approval for the world’s first 3D-printed, fully interconnected porous PEEK structure using internally developed fused filament fabrication.
The Inspire platform is manufactured using Evonik’s VESTAKEEP i4 3DF PEEK high-performance polymer on a proprietary, patented 3D printer designed, programmed and built by Curiteva.
Alex Vaccaro, MD, PhD, president of Philadelphia-based Rothman Orthopedic Institute shared, “I believe structure drives biology and the lattice PEEK architecture enabled by Curiteva’s 3D printing process represents an exciting advancement in spine, orthopedics, and neurosurgical procedures which involve any type of biologic implant”.
Kevin Foley, MD, Chairman of Semmes-Murphey Neurologic and Spine Institute and professor of neurosurgery, orthopedic surgery and biomedical engineering at the University of Tennessee Health Science Center stated, “The Inspire porous PEEK technology checks all of the boxes for an ideal interbody implant: fully interconnected porosity, modulus of elasticity equivalent to cancellous bone, strong biomechanical properties, radiolucency, and a bioactive surface for osseointegration.”
Randy Dryer, MD, Central Texas Spine Institute commented, “Interconnected porosity, pore size distribution, and nano-surface architecture are typically hallmarks of the most effective synthetic allografts. I believe this novel implant enhanced with HAFUSE nano-surface topography incorporates those features and presents an optimal environment for osteoprogenitor cells to move throughout the implant enhancing bone healing (fusion) and reducing risk of subsidence. I’m excited to offer this to my patients.”
“This new technology further demonstrates Curiteva’s commitment to developing and investing in disruptive technologies,” said Todd Reith, Inventor and Vice President of Emerging Technology. “Our unique architecture is the result of years of research and development in 3D printing. As this technology matures, with our internal expertise, we have already contemplated other applications outside of spine and orthopedics”.
The company plans a full commercial launch in the United States later this year.
Find out more about Curiteva at curiteva.com.
Subscribe to our Newsletter
3DPResso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.