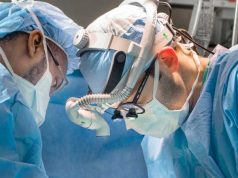
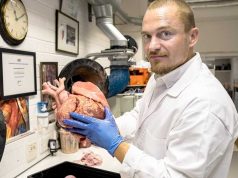
Oxford Performance Materials announced that it has received FDA clearance for its 3D printed SpineFab VBR implant system.
OPM’s SpineFab device is a vertebral body replacement (VBR) intended for use in the thoracolumbar regions of the spine to replace a collapsed, damaged, or unstable vertebral body due to tumor or trauma. To gain this FDA clearance, OPM’s VBR implant system underwent extensive static and dynamic mechanical testing to assure it meets load and fatigue requirements as well as regulatory guidelines for its intended use.
Scott DeFelice, Chief Executive Officer and Chairman of Oxford Performance Materials said: “This clearance serves as further confirmation of our ability to repeatedly build fully functional 3D-printed parts and mission critical robust structures. The introduction of our SpineFab system represents exciting news for the Company’s entry into the attractive spinal market, and this lays the foundation for future generations of load-bearing OsteoFab implants in the orthopedic industry.”
SpineFab VBR System implants will be 3D printed in 48 sizes by OPM Biomedical using only biocompatible polymer and laser light. All SpineFab implants will be manufactured by OPM utilizing the Company’s OsteoFab process, which combines OPM’s exclusive 3D printing technology with the company’s proprietary OXPEKK powder formulation to print orthopedic and neurological implants. The result is a unique and beneficial set of attributes, including radiolucency, bone-like mechanical properties, and bone ongrowth characteristics.
OPM is currently in discussions with a number of distributors regarding sales channels for its SpineFab VBR system as well as partnership options for orthopedic devices in development.
Subscribe to our Newsletter
3DPResso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.