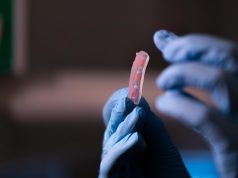
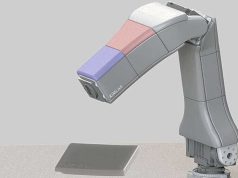
A team of engineers and doctors at the University of Minnesota have designed a unique 3D-printed light-sensing medical device that could help millions of people worldwide with lupus and other light-sensitive diseases by providing access to more personalized treatments and information to determine what causes their symptoms. The device is placed directly on the skin and gives real-time feedback to correlate light exposure with disease flare-ups.
According to the Lupus Foundation of America, about 1.5 million Americans have a form of lupus. Light sensitivity is common in people with lupus, and many find that their disease is made worse by exposure to sunlight or even artificial light indoors. The symptoms of these flare-ups for patients with lupus include rashes, joint pain and fatigue.
The research was published in Advanced Science. The researchers have also filed a patent on the device, and the technology is available for licensing.
“I treat a lot of patients with lupus or related diseases, and clinically, it is challenging to predict when patients’ symptoms are going to flare,” said David Pearson, a dermatologist in the U of M Medical School and co-author of the study. “We know that ultraviolet light and, in some cases, visible light, can cause flares of symptoms — both on their skin, as well as internally — but we don’t always know what combinations of light wavelengths are contributing to the symptoms.”
Pearson had heard about customized 3D-printing of wearable devices developed by Michael McAlpine, a professor in the U of M College of Science and Engineering and study co-author, and contacted him to collaborate on finding a solution.
McAlpine’s research group worked with Pearson to develop a first-of-its-kind fully 3D-printed device with a flexible UV-visible light detector that could be placed on the skin. The device is integrated with a custom-built portable console to continuously monitor and correlate light exposure to symptoms.
“This research builds upon our previous work where we developed a fully 3D printed light-emitting device, but this time instead of emitting light, it is receiving light,” said McAlpine. “The light is converted to electrical signals to measure it, which in the future can then be correlated with the patient’s symptoms flare-ups.”
The research team has received approval to begin testing the device on human subjects and will soon begin enrolling participants in the study.
McAlpine and Pearson said the 3D-printing process is relatively low-cost and could someday provide easy, quick access to the device without the expensive fabrication processes of traditional devices.
“There is no other device like this right now with this potential for personalization and such easy fabrication,” Pearson said. “The dream would be to have one of these 3D printers right in my office. I could see a patient and assess what light wavelengths we want to evaluate. Then I could just print it off for the patient and give it to them. It could be 100 percent personalized to their needs. That’s where the future of medicine is going.”
The research was funded by a U of M Grant-in-Aid of Research, Artistry and Scholarship grant and an Academic Investment Research Program grant. Support was also provided by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health. Portions of this work were conducted in the Minnesota Nano Center, which is supported by the National Science Foundation through the National Nano Coordinated Infrastructure Network (NNCI).
Find out more about University of Minnesota at twin-cities.umn.edu.
Subscribe to our Newsletter
3DPResso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.