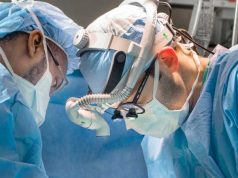
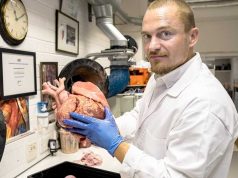
Global healthcare company Zimmer Biomet has recently received clearance from the FDA for their Unite3D Bridge Fixation System. The 3D printed system is designed to offer stability in foot and ankle joint fusion surgery and is made using Zimmer Biomet’s proprietary OsseoTi porous metal technology.
The 3D printed porous metal matrix mimics the natural architecture of bone and is designed to allow for bone ingrowth. The Unite3D system represents an alternative to traditional surgical plates, screws and staples used by surgeons in foot an ankle fusion procedures.
“The Unite3D Bridge Fixation System is unlike anything in our portfolio, and we are proud to commercialize a true innovation in this exciting clinical area,” said Ben Joseph, General Manager of Foot & Ankle. “This powerful combination of 3D printing technology and our OsseoTi porous metal material is only the latest contribution from Zimmer Biomet’s robust innovation pipeline. We aim to serve the unique needs of patients and surgeons while expanding our presence in every category of musculoskeletal healthcare, including the rapidly growing market of foot and ankle treatments.”
Subscribe to our Newsletter
3DPResso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.