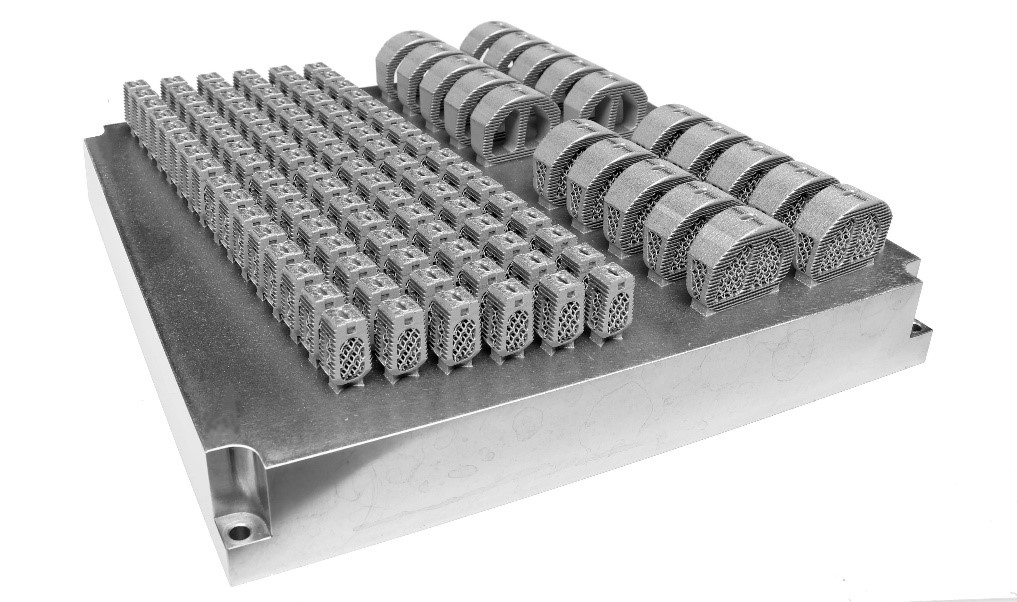
Amnovis has incorporated process improvements into its AM contract manufacturing workflow for titanium implants. This leads to significantly increased productivity, which is good news for titanium spinal cage and other medical device OEMs. Amnovis is now able to produce 3D printed spinal cages at lower cost and shorter lead times. Visit the Amnovis booth (#2627) at NASS Annual Meeting in Chicago where these 3D printed devices will be on display.
Ruben Wauthle, Amnovis CEO and Co-Founder: “For us, innovation benefits from material and process enhancements as well as the ability to increase AM productivity. By scaling our manufacturing capabilities for quality-critical applications, such as medical devices, we ensure faster delivery at lower cost, while maintaining our quality standards.”
Ruben Wauthle: “The parts manufactured using our improved workflow meet the mechanical and chemical requirements of the ASTM standards and the entire process is validated. When printing high-end products for quality-critical medical applications, we obtain a significantly higher productivity while maintaining superb quality and repeatability. The process improvements allow us to boost productivity and realize an exceptional total cost of ownership.”
Driven by the track traveled so far, Amnovis decided to shift gears to further strengthen its position in the rapidly evolving medical market. The Amnovis’ founders are AM pioneers
who were among the first to employ laser powder bed fusion (LPBF) for printing titanium
medical implants. Incorporating the process improvements into its manufacturing
workflow allow Amnovis to expand its potential for standard and patient-specific medical
device innovation.
Ruben Wauthle: “We are happy to share our competitive AM capabilities at NASS 2022
Annual Meeting in Chicago and discuss manufacturing applications for spinal cages and
other implants.”“We see great potential in innovating various AM applications across a broad spectrum of
medical device types. Highly-efficient 3D printers enable us to better align design and
development with material selection and manufacturing excellence. Our joint offering with
selected partners comprises of the complete process for customers and prospects to aim
high and move fast, while removing hurdles in medical device development, validation and
manufacturing.”“At Amnovis, we rely on our comprehensive AM workflow and production platform, which is entirely ISO 13485:2016 certified. Digital process automation enables us to provide full
traceability and repeatability to flexibly scale up manufacturing of high-end products for
quality-critical medical applications. Overall, we are maximizing productivity, reliability,
uptime and yield in our efforts to better serve titanium spinal cage and other medical
device OEMs.”
Find out more about Amnovis at amnovis.com.
Subscribe to our Newsletter
3DPresso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.