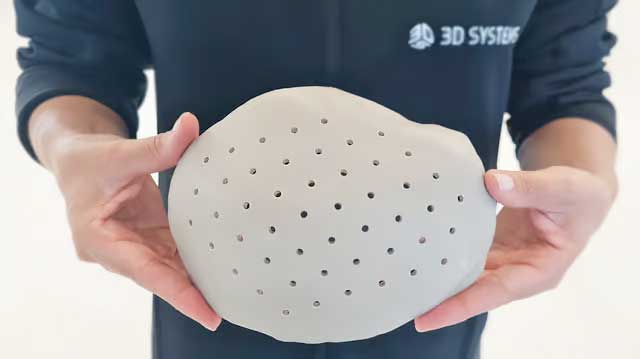
3D Systems has announced that the Food and Drug Administration (FDA) has granted 510(k) clearance for its 3D-printed, patient-specific cranial implant solution, VSP PEEK Cranial Implant.
The VSP PEEK Cranial Implant System includes a complete FDA-approved workflow chain from segmentation and 3D modeling to 3D printing. The 3D Systems EXT 220 MED 3D printer is used, which works with the high-performance polymer VESTAKEEP i4 3DF PEEK from Evonik. This material is known for its excellent biocompatibility and mechanical properties, which are similar to those of human bone.
“3D-printed PEEK cranial plates are an innovative solution that can improve patient care and expand the possibilities for precise, individualized neurosurgery,” said Dr. Johannes Pöppe, senior attending surgeon, department of neurosurgery, University Hospital Salzburg. “The solution is revolutionizing the field. The combination of 3D Systems’ printing technology that is uniquely engineered for sterile environments along with the mechanical properties of PEEK are helping surgeons push boundaries. Within our hospital, we have already completed several successful surgeries using these technologies. I believe the potential for customized PEEK cranial plates is significant to integrate 3D printing into routine clinical practice.”
The use of this manufacturing technology makes it possible to produce customized cranial implants with up to 85% less material than conventional methods, resulting in significant cost savings on expensive raw materials such as implantable PEEK. In addition, the radiolucent nature of PEEK facilitates medical imaging, giving surgeons a clearer assessment of the surgical site and implant integrity.
“As a leader in medical device innovation, 3D Systems prides itself on pioneering advancements that benefit both surgeons and patients,” said Dr. Gautam Gupta, SVP & general manager, medical devices, 3D Systems. “Receiving FDA clearance for our VSP PEEK Cranial Implant solution is a significant milestone in our journey. Our EXT 220 MED printing system has already enabled the production of nearly 40 cranial implants in support of successful cranioplasties throughout Europe. With this FDA clearance, we are now able to bring VSP PEEK Cranial Implant to the U.S. — setting a new standard of excellence for these procedures. We are now looking to the next applications for this technology, which includes 3D-printed spine interbody fusion implants, carbon fiber-reinforced PEEK for plating applications in trauma and fixation, and bioresorbable polymers for large bone and craniomaxillofacial applications.”
FDA approval enables 3D Systems to significantly expand its PEEK product portfolio with the EXT 220 MED platform, accelerating the development of highly specialized medical solutions and improving patient care. The increasing use of 3D printed cranial implants is expected to continue to grow, driven by the availability of advanced technologies and continued innovation in materials and manufacturing methods.
Subscribe to our Newsletter
3DPresso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.