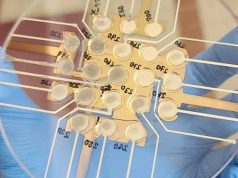
Following their announcement of establishing a 3D printing manufacturing facility this year, medtech company Stryker has received FDA clearance for their 3D printed spinal fusion device.
The Tritanium PL Posterior Lumbar Cage is an intervertebral body fusion device for lumbar spinal fixation in patients with degenerative disc disease. The devices are constructed through Stryker’s proprietary Tritanium technology and manufactured using 3D printing. The porous device is specially designed for bone in-growth.
Brad Paddock, President of Stryker’s Spine Division commented: “This is an exciting time for Stryker. We are committed to offering a full range of innovative spinal products that allow surgeons to help their patients return to a more active lifestyle. Our advanced 3D additive manufacturing capabilities allow us to precisely manufacture the porous structures of Tritanium and specific implant geometries. We are pleased to bring this technology to our spine surgeon community and their patients.”
The new implants will be available to orthopaedic and neurosurgeons in the second quarter of 2016.
Subscribe to our Newsletter
3DPresso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.