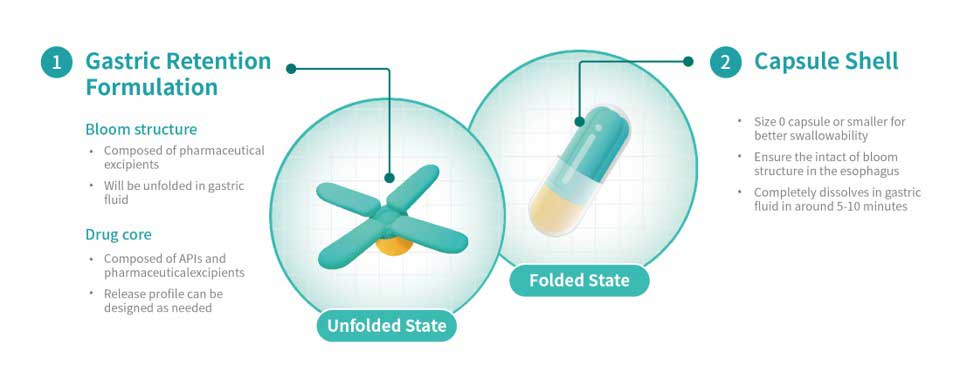
Triastek, a pharmaceutical company specializing in 3D printing, announced that the US Food and Drug Administration (FDA) has given the green light to start clinical trials with the 3D-printed gastric retention product T22. This is the first approval of a 3D-printed gastric retention product by the FDA worldwide.
T22 is a product for the treatment of pulmonary hypertensive diseases such as pulmonary hypertension. It is based on Triastek’s innovative MED&MIM technology, which combines melt extrusion with micro-injection molding. Compared to conventional dosage forms, T22 is said to have an extended gastric residence time, reducing the frequency of administration from three times a day to once a day.
According to Triastek CEO Dr. Senping Cheng, the FDA approval represents an important step in the clinical development of T22 and the underlying gastric retention technology. In addition to T22, Triastek already has three other 3D-printed drug candidates in clinical trials. This puts the company in a leading position in the field of 3D-printed drugs.
Subscribe to our Newsletter
3DPresso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.