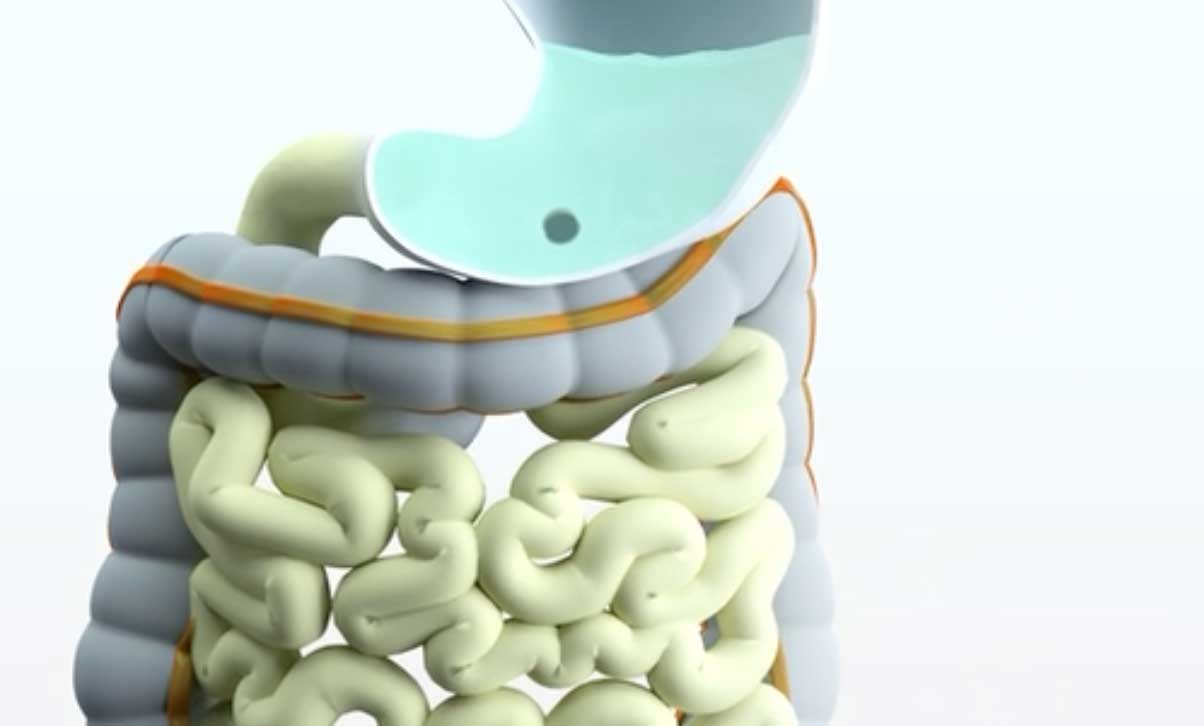
Triastek announced that it has completed a first-in-human (FIH) study of its third 3D-printed drug, T21, for the treatment of moderate-to-severe ulcerative colitis (inflammation of the colon). T21 was developed using Triastek’s 3D Microstructure for Colon Targeting (3DμS-CT) and is designed to deliver the drug directly to the colon.
Triastek’s research and development team used the technique to achieve precise delivery in the colon. To achieve this, an enteric coating and a delay layer were placed around the tablet’s active ingredient core. An imaging structure within the T21 tablet allowed researchers to track the drug’s progression through the body using X-rays.
Professor Xiaoling Li, co-founder and chief scientific officer of Triastek, said: “The first in human study data with T21 verifies the precise colon delivery capability of the MED process, and this platform is poised to become the novel drug delivery system of choice for colon targeted new product with either local efficacy or systemic absorption. We hope to continue showcasing how Triastek’s 3D printing processes can bring technical solutions to pharmaceutical companies for efficient product development of optimized drug delivery, ultimately leading to the ability to provide patients with more clinically valuable medicines.”
Triastek has developed several 3D printing-based delivery technology platforms, including 3D microstructures for sustained release, solubility enhancement and colon targeting. These techniques enable Triastek to overcome the limitations of traditional drug development and open up new production routes.
Subscribe to our Newsletter
3DPResso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.