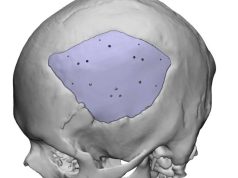
BellaSeno GmbH, a medical device company with ISO 13485 certification, has announced that it has received EU approval for its custom-made bone and pectus excavatum scaffolds.
The custom-made pectus excavatum scaffolds are among the first 3D-printed regenerative medicine products for surgical use in plastic and reconstructive surgery. BellaSeno is using its proprietary, state-of-the-art additive manufacturing platform to produce a wide range of medical scaffolds and implants. The company has already demonstrated excellent mechanical properties of its medical scaffolds, most recently at the European EFORT Congress (link). BellaSeno’s fully automated, non-contact manufacturing process enables high scalability, personalized product features and patient safety.
“We are excited about gaining European market access for our custom-made bone and pectus excavatum scaffolds,” said Mohit Chhaya, CEO of BellaSeno. “Clinical data from patients who have received the scaffolds have been very encouraging to date. We are now seeking market authorizations in other geographic regions and for additional products, such as our off-the-shelf breast implants that are suitable for both breast reconstruction and plastic surgery. Our goal is to become a diversified portfolio company and our versatile and sophisticated manufacturing infrastructure puts us in an excellent position to achieve this goal.”
“The initial results from the first patients demonstrate that our resorbable implants are safe, well tolerated and increase the potential of autologous tissue grafts,” said Dr. med. Tobias Grossner, Chief Medical Officer of BellaSeno. “We are convinced that our approach bears the potential for a paradigm change in regenerative surgery, a field that is in need of innovation to restore complex three-dimensional structures.”
BellaSeno’s ISO 13485-certified manufacturing platform is designed to meet requirements for medical scaffolds ranging from soft tissue to bone, and enables the production of both custom and sterile off-the-shelf medical implants.
Subscribe to our Newsletter
3DPResso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.