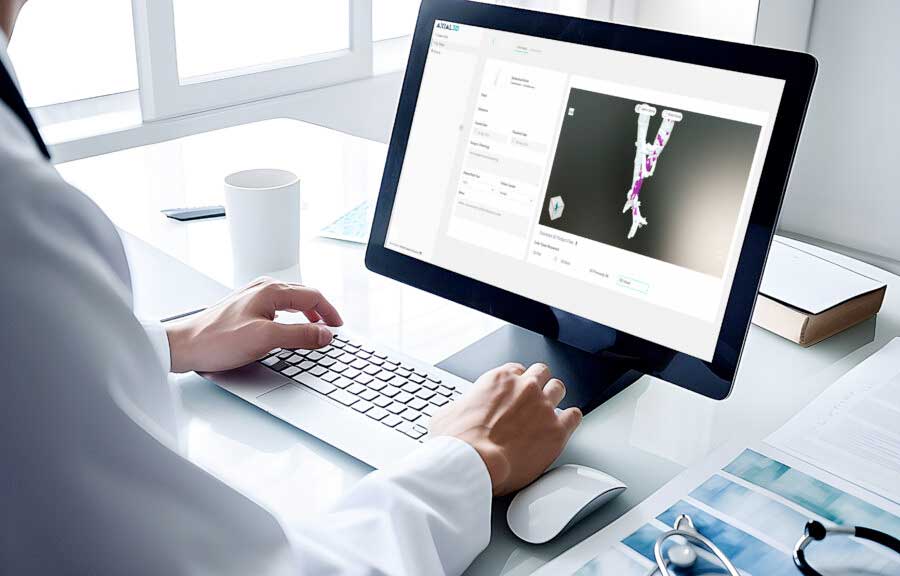
Axial3D, a provider of medical segmentation and 3D solutions, announced that it is the first company to receive FDA approval for an automated, AI-driven, cloud-based segmentation platform for orthopedic trauma, orthopedic, orthodontic and cardiovascular applications.
The platform, Axial3D INSIGHT, automatically transforms 2D DICOM images such as CT and MRI scans into accurate 3D visualizations and print-ready 3D models. This automation is designed to help healthcare providers and medical device companies save time and money.
“Our FDA clearance for Axial3D INSIGHT is a testament to how far Axial3D has come,” stated Dan Crawford, Founder and CSO of Axial3D. “From our humble beginnings as a startup to now being recognized as a leading medical technology company, this achievement showcases our dedication to pushing the boundaries of innovation in healthcare. We are immensely proud of our team’s commitment in delivering exceptional patient care using advanced automation, artificial intelligence, and machine learning technologies.”
By integrating automation and AI into its workflow, Axial3D provides medical professionals with powerful tools that facilitate access to 3D patient data. This data can then be used to improve diagnostic accuracy and treatment planning.
“Stratasys is proud to partner with Axial3D in driving innovation and in transforming the healthcare landscape with 3D solutions that help make personalized healthcare at scale possible,” said Stratasys CEO Dr. Yoav Zeif. “We congratulate Axial3D on achieving FDA clearance for their segmentation platform, leveraging the power of automation and AI as a key business driver for their continued success in the medical sector.”
Subscribe to our Newsletter
3DPresso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.