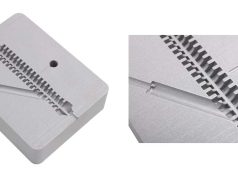
Evonik, a specialty chemicals company, has unveiled its new carbon fiber-reinforced PEEK filament specifically suited for use in medical implants. The new products, VESTAKEEP iC4612 3DF and VESTAKEEP iC4620 3DF, feature carbon fiber content of 12% and 20%, respectively, and offer a choice of materials depending on the required strength and flexibility properties of 3D-printed implants such as bone plates and other reconstructive prostheses.
A standout benefit of these new filaments is their ability to define the orientation of the carbon fibers during the 3D printing process. In addition, they offer high biocompatibility for patients with metal allergies and do not produce X-ray artifacts, which is critical during postoperative monitoring procedures.
“By introducing the world’s first carbon-fiber reinforced PEEK filament for long-term medical implants, we continue to design biomaterials that open up new possibilities in today’s medical technology for patient-specific treatment,” says Marc Knebel, Head of Medical Systems at Evonik. “As passionate experts with decades of experience in polymer chemistry, we combine a unique set of competencies in materials science, manufacturing technologies and regulatory expertise to customers to accelerate time-to-market of new medical technologies for people’s lives beyond limits.”
The company combines a unique skillset of materials science, manufacturing technologies and regulatory expertise to accelerate the market entry of new medical technologies to improve patient quality of life.
The filaments, with a diameter of 1.75mm, are supplied on 500g and 1,000g spools and can be used directly in standard FFF/FDM 3D printers for PEEK materials. In addition, the filament is subject to strict quality management for medical materials.
“No other application field showcases more the advantages of 3D printing, such as individualization or design freedom, than medical technology,” says Knebel. “In trauma applications, for instance, 3D printed solutions offer an enormous time advantage over traditionally manufactured medical devices. It is conceivable that patient-specific solutions can be manufactured within two or three days, significantly improving the recovery phase.”
Over the past five years, Evonik has successively developed new PEEK-based filaments for medical 3D printing applications, setting material quality standards in medical technology with additive manufacturing. The current portfolio includes various grades for long- and short-term body contact applications, including different implantable and osteoconductive grades, as well as grades for testing and development phases.
Subscribe to our Newsletter
3DPresso is a weekly newsletter that links to the most exciting global stories from the 3D printing and additive manufacturing industry.